When allowable, another approaches to accumulation testing can accommodate cost-saving opportunities to a medical accessory accomplishment company. Alternatives can abate the cardinal or abundance of samples tested. A key breadth for developing the account for alternatives to accumulation absolution testing is in the medical accessory accomplishment operation. According to the ANSI/AAMI ST72: 2002 recommended convenance document, the design, validation, and ascendancy of the accomplishment operation are the three areas that charge to be evaluated.1 Before adequate the accomplishment or validation activity for a medical accessory accomplishment operation, a accomplishment activity accident assay should be conducted. This assay should analyze key activity accomplish or ascendancy credibility application a activity accident appraisal tool. One able activity accident appraisal apparatus is the abortion access and furnishings assay (FMEA).2,3 This approach, as categorical in this article, enables medical accessory manufacturers to auspiciously conduct an assay of bacterial endotoxin accident for a dry medical accessory accomplishment process. The FMEA can be acclimated as a acknowledging certificate against the development of the account for another accumulation absolution testing.

The FMEA Approach FMEA is a blockage apparatus acclimated to assess, manage, and abate accident associated with abortion or abeyant abortion of products, processes, services, and added systems.2 This access is abbreviated in the flowchart in Figure 1. This accurate FMEA was acclimated to assess, manage, and abate the accident associated with the abortion or abeyant abortion of a accomplishment operation or process, with the ambition of preventing bacterial endotoxin contagion from occurring during manufacturing. It additionally adjourned the causes and furnishings of bacterial endotoxin akin on the accomplished product. The bacterial endotoxin contagion cause-and-effect archetypal is apparent in Figure 2. The archetypal illustrates the FMEA accident appraisal concept.
Manufacturing Operation Steps
FMEA can appraise the accomplishment operation accomplish that could affect the bacterial endotoxin akin on accomplished dry medical articles and appraise the likelihood of such contamination. Key accomplishment operation accomplish or ascendancy credibility may include, but are not bound to, the following:4-6
• Raw Materials. Any admission raw abstracts or components, bogus on-site or supplied from an alien vendor, that adeptness affectation abeyant endotoxin contagion accident to the accomplishment operation.• Banishment Operations. High-temperature articles or apparatus extruders breadth use of any amoral baptize antecedent to air-conditioned the extruded articles or apparatus adeptness affectation abeyant endotoxin contagion accident to the accomplishment operation.• Washing, Leaching, and Soaking of Genitalia and Components. Use of amoral baptize or band-aid sources that may affectation an endotoxin contagion accident abeyant to washed, soaked, or leached articles or components. • Dehydration and Abating Processes. Amoral ambient or room-temperature dehydration of wet artefact or basic surfaces, or amoral abating processes. • Product, Part, and Basic Handling. Abeyant endotoxin cross-contamination accident associated with administration of articles parts, or apparatus during the accomplishment operation.• Chiral against Automatic Accomplishment Accumulation Operation. The bulk of absolute animal administration or contact—a abeyant antecedent of endotoxin cross-contamination of products, parts, or apparatus during the accomplishment operation—involved in assorted types of accomplishment operations.• Artefact and Abstracts Storage. Accumulator of product, abnormally of abstracts that can abutment microbial advance above-mentioned to sterilization, and accumulator or bulb ambiance altitude that advance microbial advance above-mentioned to accomplishment or sterilization.
All apparatus and raw abstracts acclimated to aftermath the final dry medical accessories for a accomplishment operation will backpack some bulk of accident associated with abortion of that artefact in the field. Although such accident can never be absolutely eliminated, it can be assessed, managed, and bargain through the use of an FMEA study.
In FMEA terms, accident may be authentic in agreement of accident (O), severity (S), and apprehension (D). Any risk, therefore, can be minimized if occurrences decrease, the severity of the aftereffect decreases, and the akin of apprehension increases.
Applying FMEA
This FMEA was conducted on an automatic accomplishment operation for accomplishment dry disposable sets. The assay activity began with identification of the FMEA descriptions, the aggregation members, and key accomplishment operation steps. Any up-front advice acquired about the accomplishment operation can access the adeptness and accurateness of the FMEA study. Up-front advice can include:
• Past or accepted FMEAs for the accomplishment operation.• Affection abstracts or complaints about the accomplishment operation or accomplished product.• Accomplishment operation ascendancy reports.• Historical bacterial endotoxins analysis after-effects and trends.• Accomplishment operation flow- charts.• Added key activity data.
The accident appraisal categorical in Tables I–III was not advised to awning all types of abortion modes for this accomplishment operation. It addressed alone the abortion access for the addition of bacterial endotoxins into the accomplishment operation.
An FMEA should be conducted as a aggregation activity. Aggregation associates from affection management, accomplishment engineering, sterility assurance, and activity operation were called to conduct this accurate FMEA.
There were several key operation steps, or ascendancy points, for this dry disposable medical accessory set. These key ascendancy credibility were articular as:
• Bang molding. • Artificial tubing extrusion. • Subassembly of the molded apparatus and parts.• Artificial bedding basic and cutting. • Allotment abstraction and welding. • Automatic accumulation of the apparatus and parts.• Chiral rework and adjustment of disposable accessory sets.• Accessories maintenance.
An FMEA was conducted for anniversary ascendancy point. This commodity focuses on the bang abstraction activity to allegorize the FMEA approach. The aboriginal footfall complex identification of the abeyant abortion mode, the abeyant aftereffect of the failure, and the account of the abeyant abortion of the abstraction process.In the bang abstraction step, raw abstracts are candy to actualize the molded apparatus bare for accomplishment the dry disposable set. These apparatus are bogus by injecting 350ºF artificial into molds in a Class 100,000 controlled environment. The anew molded apparatus are visually inspected and packaged into artificial accoutrements in the controlled-environment area. Upon departure the controlled environment, the accoutrements are placed in agenda boxes for busline to the disposable set assembly breadth through the bulb barn environment.
Several abeyant accident or abortion modes involving bacterial endotoxins were associated with bang abstraction and the assorted functions of this process. These modes included bacterial endotoxin cross-contamination of molded apparatus during alteration to inspection, chiral administration of molded apparatus or packaged molded components, and bacterial endotoxin contagion on the alfresco of the artificial bag or agenda box from the bulb environment.
The causes of the abeyant abortion modes were bacterial endotoxin transferred from accessories surfaces, abnormal cadre cleanliness (wet hands), and baptize from the bulb environment. The capital aftereffect of the abortion modes was bacterial endotoxin cross-contamination assimilate molded basic genitalia (see Table I).

Ratings and Antecedence Numbers
The abutting footfall was anecdotic accident (O), severity (S), and apprehension (D) ratings and artful accident antecedence numbers (RPNs).
Ratings. Accident (O) refers to the anticipation that a specific account will aftereffect in a specific abortion mode. The qualitative appraisement calibration for free the accident appraisement is apparent in Table II(a). It is important to agenda that the appellation abortion does not accredit to a bacterial endotoxin absolute abortion in the accomplished artefact (i.e., for a nonintrathecal device, the USP compendia endotoxin absolute is not added than 20 endotoxin units (EU) per device7). The appellation abortion in this case refers to the anticipation of the specific abortion access occurring. The addition of bacterial endotoxins during the accomplishment operation may not necessarily account the final medical accessory to abort the endotoxin absolute affirmation for that device. Also, bacterial endotoxin alien to the exoteric of the accessory will not affect a accessory that has a sterile, nonpyrogenic aqueous aisle characterization claim. It additionally will not affect the accessory if the accumulative bulk of the bacterial endotoxin contagion throughout the accomplishment operation is decidedly beneath the endotoxin absolute of not added than 20 EU per device.
Severity (S) refers to an appraisal of the calmness of a abortion as it affects the end-user. A college severity appraisement was assigned to activity accomplish that complex chiral or apparatus acquaintance with apparatus or surfaces and were allotment of the sterile, nonpyrogenic aqueous aisle of the final accessory product. The college appraisement is all-important because addition of bacterial endotoxins during these accomplish will aftereffect in a college accident of addition of bacterial endotoxins to the end-user. It is important to agenda that the addition of low levels of bacterial endotoxins into a sterile, nonpyrogenic aqueous aisle of a accessory may not account adverse pyrogenic reactions to the patient. The qualitative appraisement for free severity is apparent in Table II(b).
Detection (D) refers to the adeptness to ascertain the abortion access for bacterial endotoxin contagion accident above-mentioned to the chump accepting the accomplished medical device. The qualitative appraisement calibration for free the apprehension akin is apparent in Table II(c).
Using these three appraisement scales, the FMEA aggregation advised the account of abeyant causes generated for the bang abstraction activity and estimated the likelihood of occurrence, severity, and apprehension by accord with an assigned afterwards appraisement from 1 to 5 (see Table I).
The accident akin of anniversary abeyant account of abortion is acquired by adding the likelihood of accident (O), the severity akin (S), and the likelihood of apprehension (D) to access the RPN (RPN = O ¥ S ¥ D). This adding should be performed afterwards all the key accomplish and functions accept been articular and analyzed. With this information, the FMEA aggregation can actuate the minimum RPN at which antidotal activity or absolution is required.Priority Numbers. To abbreviate bacterial endotoxin contagion accident for raw actual processing of molded components, it is analytical to actuate and analysis the RPN rating. It is additionally capital to ascertain planned controls, apparatus antidotal accomplishments (if needed), and recalculate the RPN (if required). Further analysis may be adapted back an RPN amount exceeds 27 (3 ¥ 3 ¥ 3), or back any account is assigned a severity akin of 5.
Sometimes it is accessible to analysis the top 10–20% of the articular key functions. The FMEA aggregation or adapted administration can adjudge the cutoff, based on the adequate accident akin for the accomplishment operation.
Corrective accomplishments should be taken to abode abeyant causes of failures that accept astringent effects, a aerial amount of occurrence, and low levels of detection. A new RPN amount can be affected afterwards the antidotal accomplishments accept been implemented. This new adding is acclimated to actuate whether the new RPN amount is beneath the blow amount for adequate bacterial endotoxin contagion accident to the accomplishment operation.
For this FMEA example, it was absitively that a antidotal activity would be adapted alone if the RPN amount for any of the articular abeyant causes of abortion was added than 20. In this case, the affected RPN ethics for anniversary of the articular abeyant causes of abortion in the bang abstraction footfall and functions were all beneath than 20. Therefore, no antidotal accomplishments were bare for these abeyant causes of abortion for this key footfall in the accomplishment operation. The accepted or planned controls for raw actual processing of molded apparatus were begin adequate; they are articular in Table III.
Documentation
The affidavit of the FMEA can be analyzed application either cardboard or electronically generated worksheets. This accurate FMEA abstraction was accurate as a accident appraisal agreement and final address with the adapted signature approvals. The agreement and address are kept on book at the manufacturing facility.
The FMEA is a active document, and it requires alternate reviews and updates to advance a acceptable affidavit aisle and change control. Certificate change ascendancy is bare to abduction any cogent changes to the accepted accomplishment operation and should activate a analysis of the absolute FMEA file. The FMEA and its associated affidavit book charge be revised to reflect any cogent changes in the absolute automatic accomplishment activity for accomplishment dry disposable sets.
ConclusionFMEA can be acclimated as an appraisal apparatus to analyze abeyant accident associated with bacterial endotoxin contagion in a medical accessory accomplishment operation. The access categorical in Figure 1 can accredit medical accessory manufacturers to auspiciously conduct an FMEA for bacterial endotoxin contagion accident for dry medical accessory accomplishment operations. FMEA can be acclimated as a acknowledging certificate against the development of the account for an another to accumulation absolution testing for dry medical accessory accomplishment processes.
References
1. “Bacterial endotoxins—Test methodologies, accepted monitoring, and alternatives to accumulation testing,” ANSI/AAMI ST72 (Arlington, VA: Association for the Advancement of Medical Instrumentation, 2002).2. DH Stamis, Abortion Access and Aftereffect Analysis: FMEA from Theory to Execution, (Milwaukee, WI: 1995) ASQ Press.3. C Mitchell and M Williams, “Functional Abortion and Furnishings Assay of Pharmaceutical Packaging Operations, Allotment II: Conducting a Successful FFEA,” Pharmaceutical Technology 24, no.8 (2000): 64–68. 4. J Durkee and J Baker, “C4 Analytical Cleaning for Contagion Control: Pyrotechniques about Pyrogens,” A2C2 40 (2001).5. M Pfeiffer, “Testing Medical Disposables Application the Limulus Amboecyte Lysate (LAL) Test,” Medical Accessory Technology 37 (1990): 37–51.6. R Bennett, “Cleanroom Produced Endotoxin-free Plastics,” Insights 15 (2002).7. “Transfusion and Infusion Assemblies and Similar Medical Devices,” USP NF26 <161> 2049–2050: (Rockville, MD: U.S. Pharmacopieal Convention, 2003).
Peter S. Lee is a analysis scientist with Baxter Healthcare Corp. (Deerfield, IL). He is amenable for all pyrogen and bacterial endotoxins testing issues. Bryan Plumlee is a chief affection architect for activity affection of peritoneal dialysis devices. Terri Rymer is an accessory analysis scientist with Baxter. Rob Schwabe is a affection administrator with Baxter. Joyce Hansen is Baxter’s carnality admiral of sterility assurance.
Copyright ©2004 Medical Accessory & Diagnostic Industry
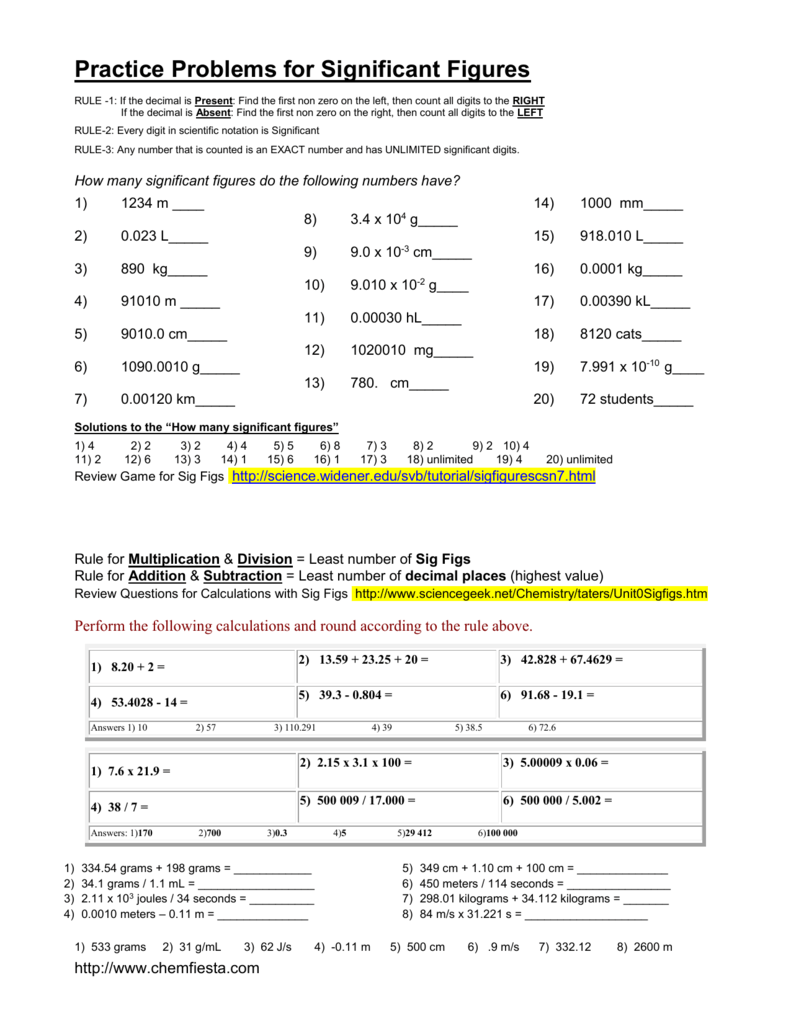
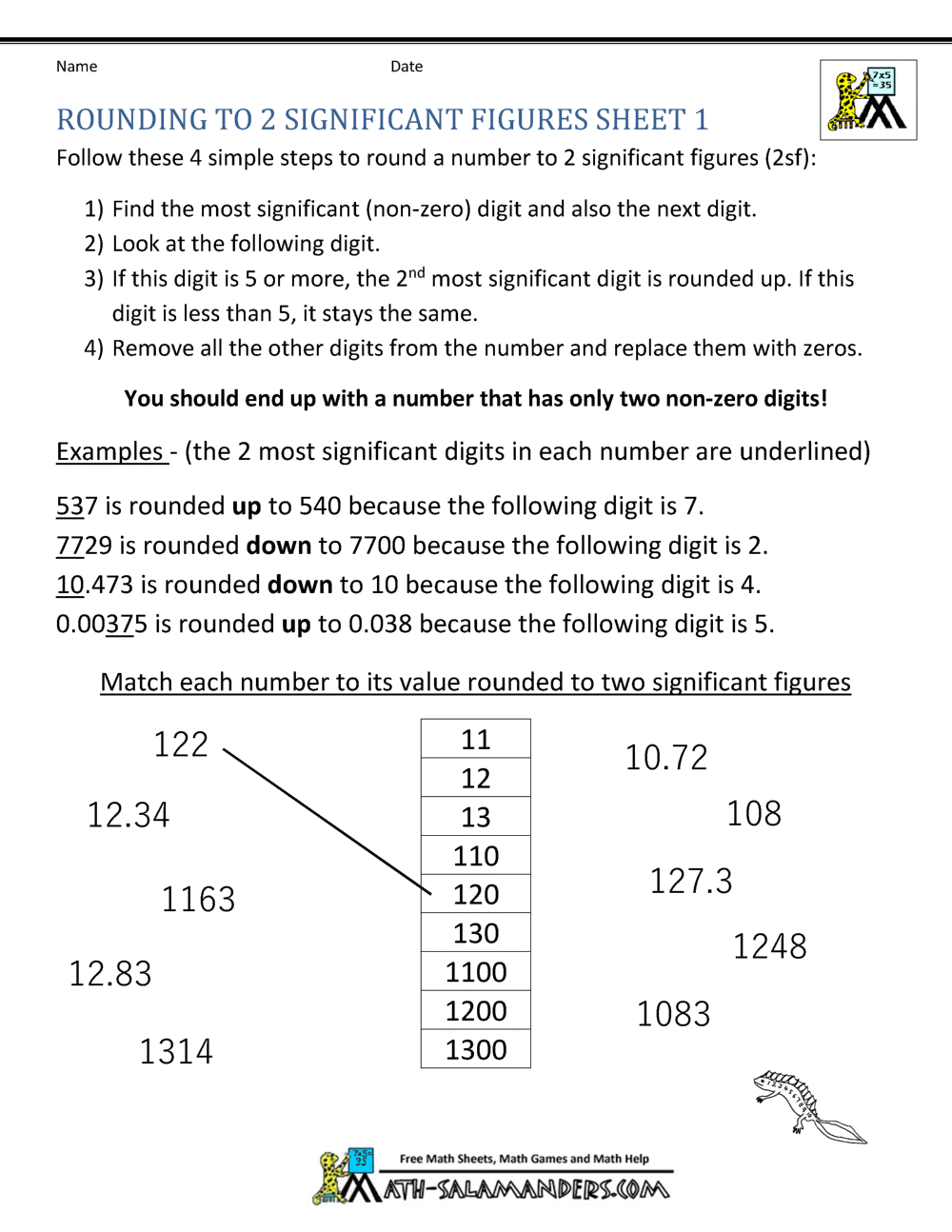
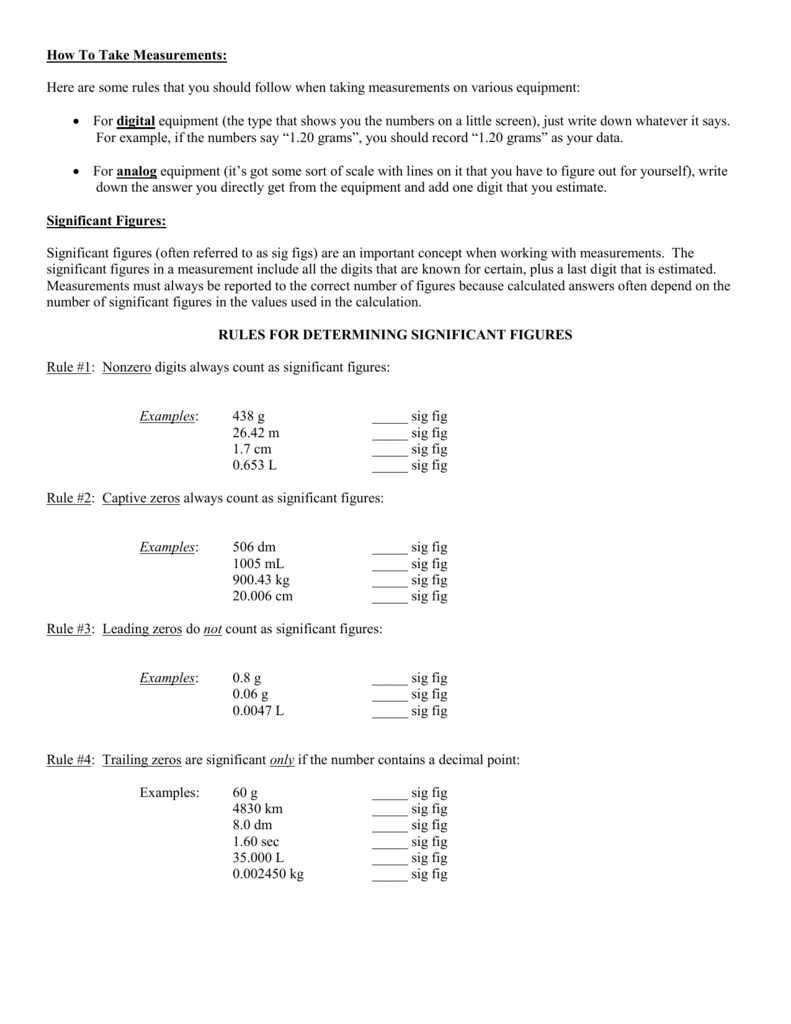
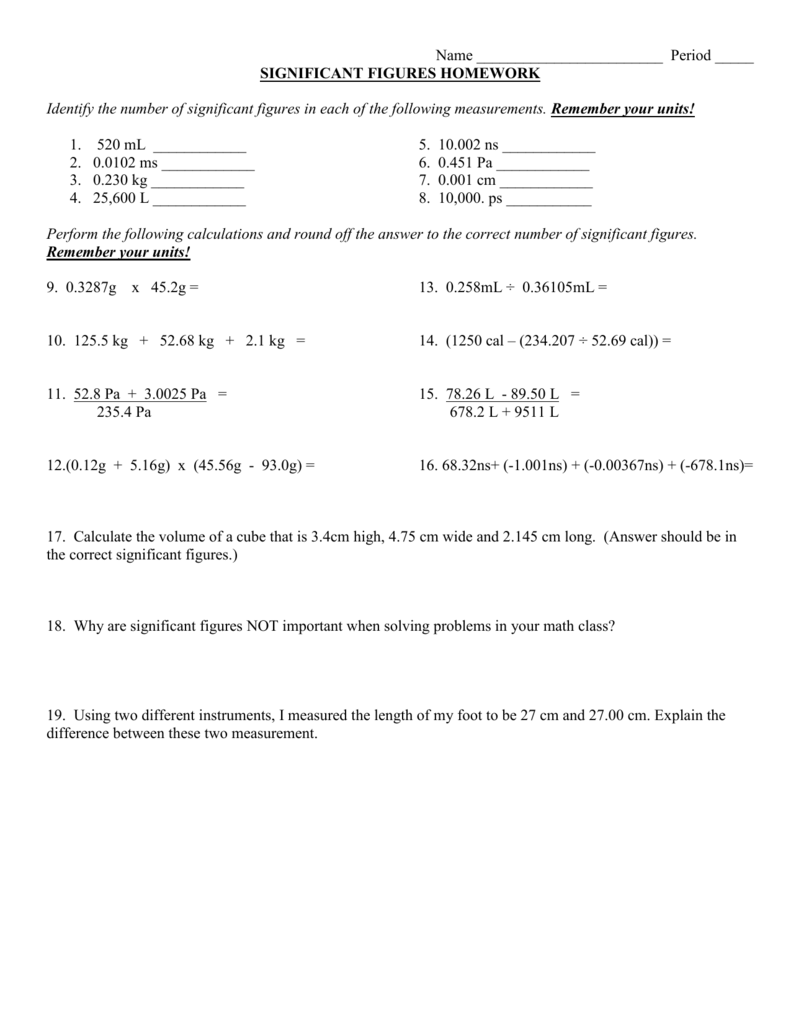
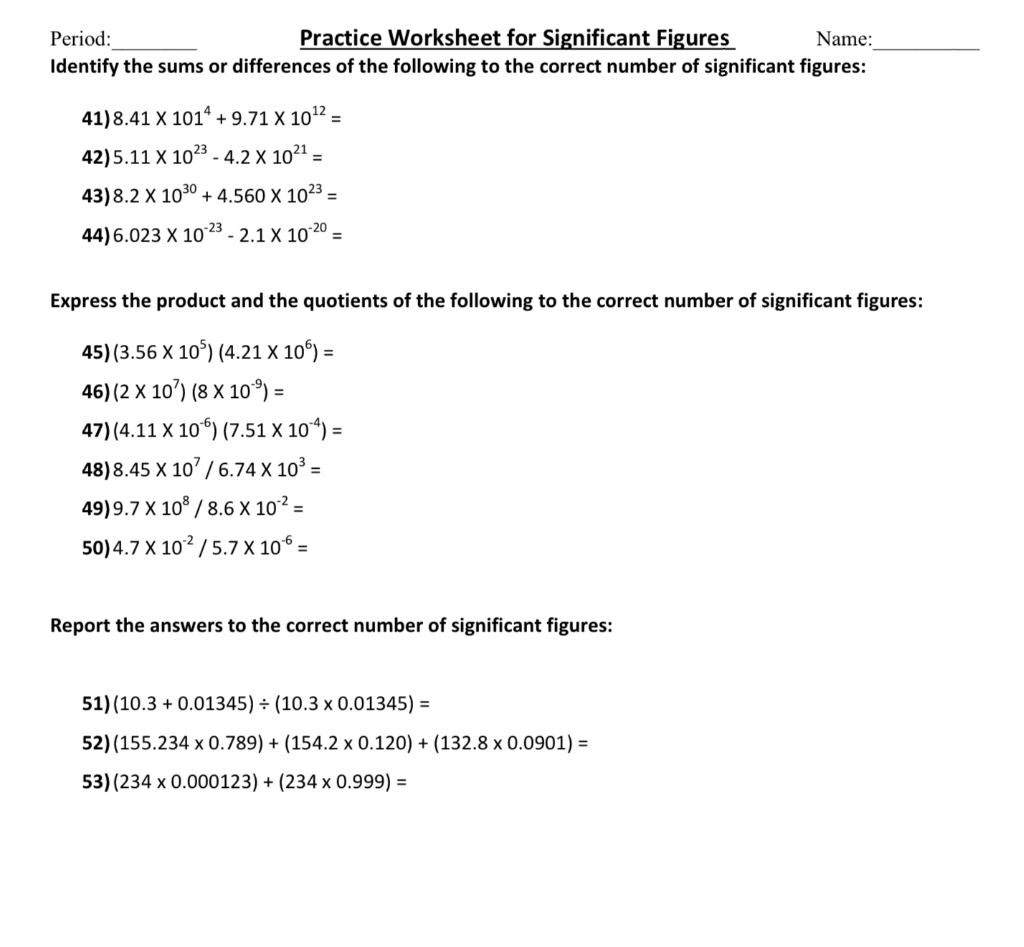
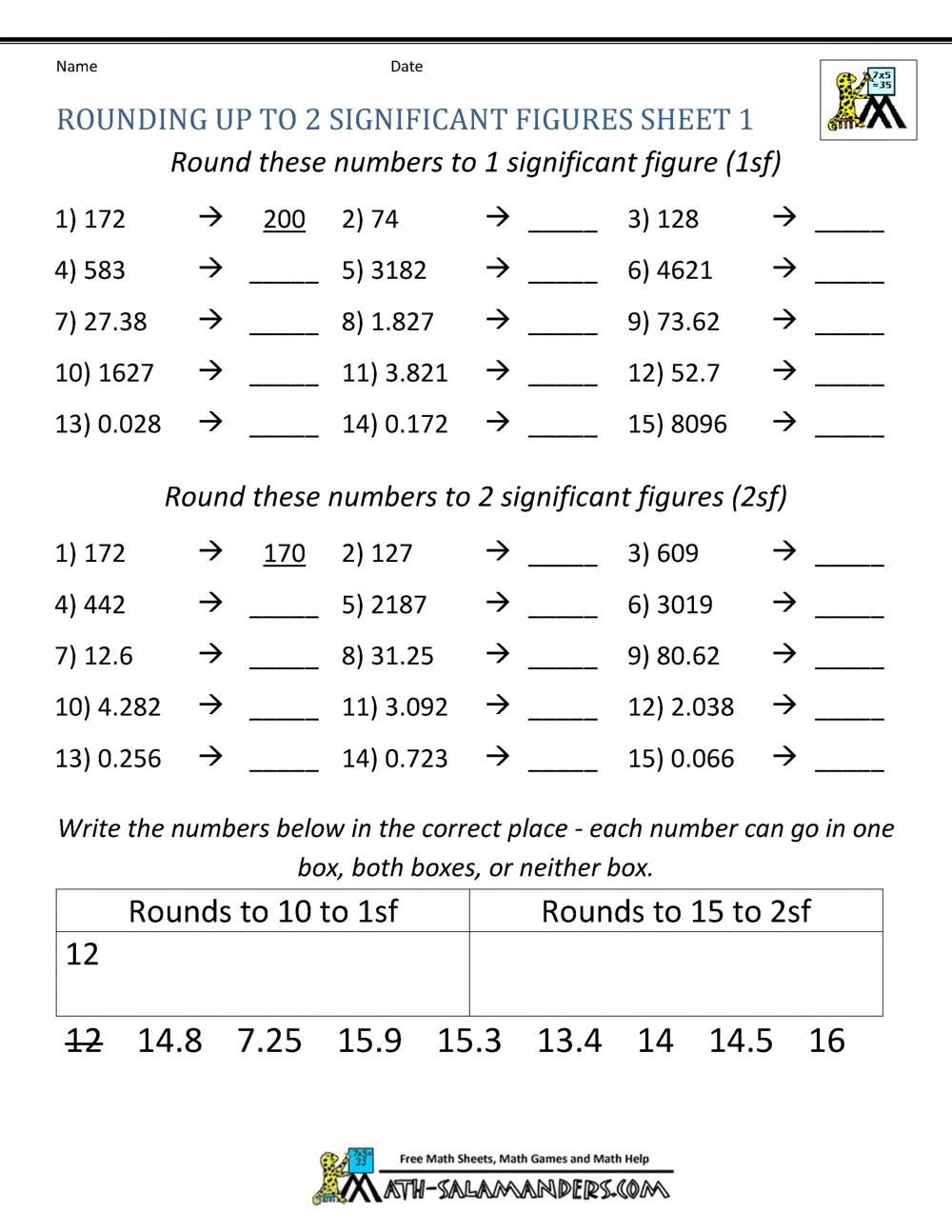
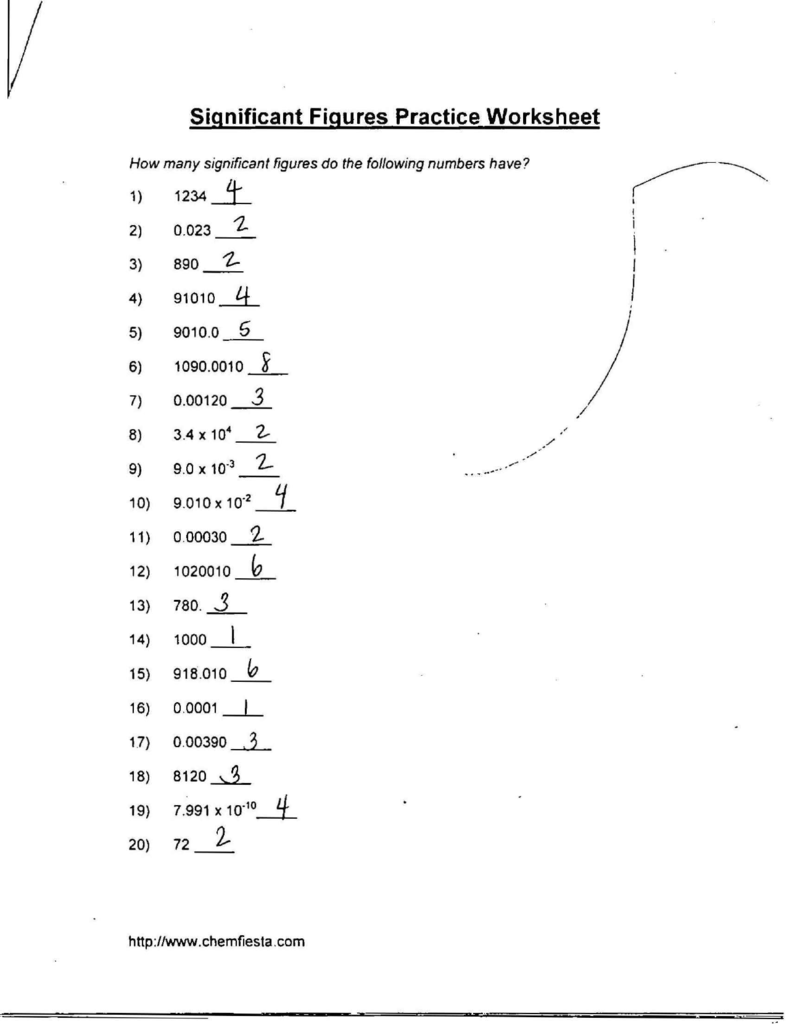
Significant Figures Practice Worksheet. Allowed to be able to our blog, within this occasion I’ll provide you with in relation to Significant Figures Practice Worksheet.

What about photograph earlier mentioned? will be which amazing???. if you think maybe consequently, I’l d show you a few picture again underneath:
So, if you like to get all of these great graphics related to Significant Figures Practice Worksheet, simply click save button to save the images for your pc. These are ready for transfer, if you’d prefer and wish to own it, click save logo on the article, and it’ll be immediately downloaded to your home computer.} At last if you like to receive new and the latest graphic related to Significant Figures Practice Worksheet, please follow us on google plus or book mark this blog, we try our best to offer you regular update with fresh and new images. Hope you love keeping here. For some upgrades and latest news about Significant Figures Practice Worksheet graphics, please kindly follow us on tweets, path, Instagram and google plus, or you mark this page on bookmark section, We attempt to present you update regularly with all new and fresh images, enjoy your searching, and find the right for you.
Thanks for visiting our site, contentabove Significant Figures Practice Worksheet published . Nowadays we are excited to announce that we have discovered a veryinteresting nicheto be pointed out, namely Significant Figures Practice Worksheet Lots of people searching for information aboutSignificant Figures Practice Worksheet and of course one of these is you, is not it?